I thought I would post something different today! While cleaning up the other day, I found a few of my college reports and had a fabulous time reminiscing the good old days when all I would think about day in and day out was Marine Biology. I remember writing this paper and visiting the Natural History Museum of Los Angeles to photograph the Catalina megamouth shark specimen.
To make things a bit easier to read, I have broken up the body of my report to make it flow better. Anyone familiar with Scientific Papers can be chunky, so I hope this helps.
When I composed this report, only four megamouth sharks were ever seen. Over 30 years, 58 more can be added to that list. So please remember while you are reading this that, scientifically, this paper doesn’t hold much faith due to its age.
But, most of the information is still very relevant, especially the morphology. Have fun, and check out the end, as I will list all megamouth sharks that have been observed so far.
Table of Contents
Introduction- Megachasma pelagios, Family Megachasmidae

A rare and startling discovery occurred on November 15, 1976, off Oahu, Hawaii. A U.S. Navy research vessel was anchored at about 165 m depth and had a strange 4.5 m adult male shark attached.
When they brought the shark onto the ship, they had a pretty good idea that this was a shark that no one knew. The crew of the AFB-14 dubbed the creature “Megamouth” because of its big rubbery jaws and large oral cavity (Clark, 1981).
Soon after the discovery, the shark was brought to the NOSC (Naval Ocean Science Center), and Dr. Leighton R. Taylor made a preliminary examination of Honolulu’s Waikiki Aquarium (Wood, 1986). Taylor suggested that it was a distinct undescribed species.
The Megamouth shark went without a scientific name until 1983, when the official description was finally published (Taylor, Compango, and Struhsaker, 1983).
A New Species of Shark?
This discovery constituted a new species and genus (Megachasma pelagios) and a new family within the order of Lamniformes (Megachasmidae).
Taylor et al. (1983) determined that the megamouth is a filter-feeder, and they compared it with the basking shark (Cetorhinus maximus), one of the two other known filter-feeding sharks.
They pointed out, however, that no other known shark has a jaw structure, snout, gill rakers (formed from dermal papillae), and dermal grooves on its fins like those found on the megamouth (see Table 1).
Only Four Megamouth Specimens Were Observed From 1976 to January 1989
Since the discovery of the first specimen in Hawaii (Fig 1 &2), three other male specimens have been discovered.
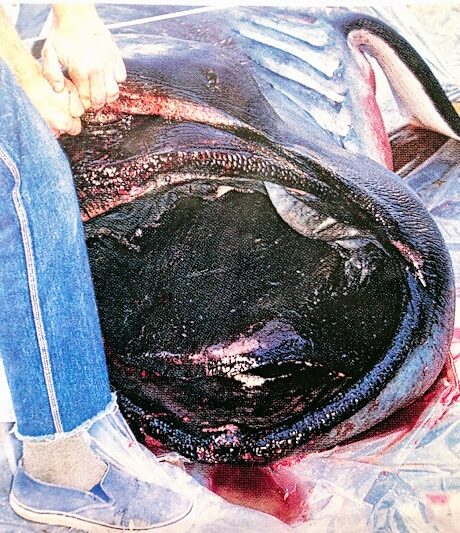
- On November 29, 1984, a megamouth shark measuring 4.5 m was caught in a gill net of a commercial fishing boat Helga at 38 m off Santa Catalina Island, California. The specimen is now displayed at the Natural History Museum of Los Angeles County (Lavenburg and Seigal, 1985).
- A third specimen, 5.15 m long, was washed up alive on a beach in Mandurah near Freemantle, Western Australia, on August 18, 1988. It was collected and preserved intact by the Western Australian Museum (Berra and Hutchins, 1990)
- A fourth specimen, estimated to be more than 4 m in total length, was found stranded on the sandy beach of Hamamatsu City in Shizuoka Prefecture, Japan (Nakaya, 1989). They could not capture the specimen because it was washed away soon after discovery.
The discovery of these four specimens has caused much excitement in the scientific world and left many unanswered questions regarding behavior since no live specimen has been studied extensively.
Although it must be noted that in the late 80s, one specimen was captured and then released after being videotaped, to my knowledge, no scientific literature has been published on the event.
Also, the Australian specimen was seen the day before two surfers beached it. They reported that they examined it in shallow water and thought it was a whale trying to beach itself, so they decided to coax it back into deeper water.
I wish to concentrate on two main aspects: the phylogenetic relationship of the megamouth shark with other Lamniformes and the other several feeding scenarios given by various authors.
Table 1- General Morphology of the Hawaiian Specimen
(Taylor et al., 1981)
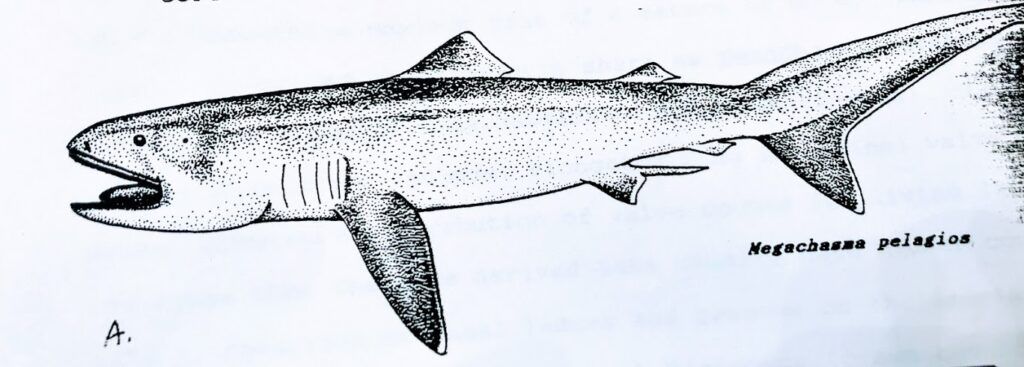
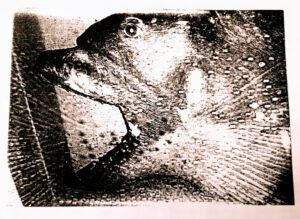
Figure 3
- A. Megamouth shark Megachasma pelagios based on the holotype
- B. Catalina specimen showing jaw protrusion (Natural History Museum Los Angeles County, 1991)
Trunk– cylindrical, tapering rearward from the head
Caudal peduncle– short, stout, slightly compressed; lacking keels or ridges
Head– broad, very large and long, not pointed; length greater than abdomen between pectoral and pelvic bases
Snout- very short and depressed
Eyes– lateral on the head; length less than one-fourth of the length of the longest gill openings
Nostrils- small; lateral and opposite the first fourth to mouth
Gill Openings- moderately large, not expanded onto the dorsal surface of the head
Internal gill openings– numerous gill rakers of a unique type formed as elongated, slender, cartilage-cored; dermal papillae covered by imbricated denticles
Mouth- terminal and very large, extremely long and extending far behind eyes when jaws are not protruded
Jaws- strongly protrusible, capable of extension well in front of snout; inner labial grooves present along edges of mouth corners
Teeth- similar in upper and lower jaw; weekly differentiated; not compressed or blade-like; relatively small; and very numerous; over 100 rows in each jaw, and in 3 of 4 functional series
Lateral trunk denticles- broad tear-drop or wedge-shaped; flattered crowns, not erect, hooked, or directed anteriorly or dorsal- ventrally; wavy grooves of naked skin on pectoral, pelvic, and caudal fin webs
Pectoral fins- relatively narrow, long, and blunt-tipped; origin under fourth-gill openings; skeleton plesodic, with pectoral radials extending into the distal fin web nearly to its edge; ceratotrichia reduced along the distal fin margin and not expending proximally to radial musculature of fin
Claspers– moderately slender and elongated, with attenuated tips and external spurs
First dorsal fin– moderately large, angular, and relatively low; origin much closer to pectoral fin bases than pelvic bases and free near the tip, well in front of pelvic origins
Second dorsal fin– less than one-third area of the first dorsal and slightly less than half as high; origin about over the pelvic fins insertions
Anal fin- about half area of second dorsal and it is free near tip well in front of the ventral caudal origin
Caudal fin– with a long dorsal lobe nearly half-length of rest of shark; a long ventral lobe about 2/5 as long as a dorsal lobe; not lunate of crescentic; dorsal, caudal vertebrae axis moderately elevated at an angle to the body axis (heterocercal)
Megamouth Relationships With Other Lamniformes

According to the definition given by Compango (1981), Lamniformes or Lamnoid sharks are “typical sharks” with large mouths, conical or flattened snouts, two dorsal fins without spines, and anal fin, eyes over the mouth, and nostrils separate from the mouth.
Almost all lamnoids are large or very large sharks reaching a maximum size of 4 meters or more.
Taylor et al. (1983) described the megamouth shark as Megachasma pelagios in the monotypic family Megachasmidae (Order Lamniformes).
Lamnoid-derived characters include:
- elongated ring intestinal valve (turns/valve- frequency distribution of valve counts for living lamnoid, indicated that the more derived taxa usually have higher counts [27] ),
- reduction of basal ledges and grooves on the labial crown of the teeth; osteodont tooth histotype (crown having a core of osteodentine and no pulp cavity or canal [Compango, 1988] );
- absence of subocular ridges between the eyes and jaws (therefore, jaws are highly protrusible);
- reduced labial cartilages of the jaw (find inner labial groove present along the mouth instead);
- vertebral calcification pattern with centra poorly calcified (notochord sheath very wide between the center and primary and secondary calcification is virtually absent).
- Taylor et al. (1983) also noted that the megamouth shark shared derived plesodic pectoral fins (the distal radials are greatly elongated and support the fin web) with the advanced lamnoid families, Alopiidae, Cetorhinidae, and Lamnidae.
- They also noted that megamouth teeth are superficially similar to the other lamnoid filter-feeder, the basking shark (Cetorhinus maximus, family Cetorhinidae).
It was suggested by Taylor et al. (1983), as an alternative to placing Megachasmids with the advanced lamnoids, that Megachasmidae might be the primitive sister group of all living lamnoids.
This assumption was based on the presence of a strong palatoquadrate orbital process and the absence of differential tooth row groups in M. pelagios, which was thought to be primitive relative to other lamnoids.
Also, Taylor et al. (1983) suggested that the simple dentition of the megamouth shark might be secondarily reduced, correlated with its functional replacement by gill rakers.
Maisey (1985), rejected the placement of Megachasma as the sister group of all other lamnoids but was convinced that plesodic pectorals united Megachasma with the advanced lamnoid families.
Maisey (1985) suggested that the megamouth was confamilial with the basking shark because of the synapomorphies in their jaw suspension, cranial morphology, dentition, and filter-feeding structures.
“Cetorhinus and Megachasma seem to form a monophyletic group of filter-feeding lamniformes” (Maisey, 1985).
This hypothesis was later rejected by Compango (1990). In his analysis between the basking shark and the megamouth shark, he indicated that they were not a monophyletic group of specialized filter-feeding lamnoids.
He believes that the Megachasma filtering system is derived from the more primitive arrangement in the Odontaspididae. At the same time, that of Cetorhinus is derivable from the arrangement found in the Lamnidae.
Therefore, filter-feeding can not be considered a synapomorphic character of Cetorhinus and Megachasma (Compango, 1990).
Further analysis done by Compango (1990) indicated that the lamnoids with plesodic pectoral fins form a derived group, but that of Megachasma has primitive characters found in aplesodic lamnoids.
A New Family of Sharks- Megachasmidae
Compango believes this makes the family Megachasmidae the plesiomorphic sister group of the Alopiidae, Cetorhinodae, and Lamnidae.
These characters include:
- its low first dorsal fin with aplesodic skeleton (distal radials not extending into the fin web),
- low intestinal valve count (27 with >30 being considered more advanced)
- highly protrusible jaws
- and probably also the odontaspidid-like size, shape, and spacing of its dorsal, anal, and pelvic fins (see Fig 3).
Therefore, Compango (1990) feels that these features support the separation of the Megachasmidae and Cetorhinidae and rejects the hypothesis that the megamouth shark is the primitive sister of all other lamnoids.
Megamouth Feeding Scenarios
One aspect of the megamouth shark of particular interest is its feeding habit, which is considered unique among all other animals. Among the 300 species of sharks, Megachasma pelagios is one of the only three that are filter-feeders.
The stomach of the Hawaiian specimen contained 1-inch long euphausiid shrimps (Taylor et al., 1983), and the Catalina specimen had shrimps and deep-water jellyfish (Lavenburg and Seigal, 1985).
Comparing the Megamouth to the Basking Shark and the Whale Shark

In habitat and anatomy, the megamouth differs greatly from the other filter-feeding sharks (Diamond, 1985). The basking shark swims with its mouth open near the surface of the cold, nutrient-rich coastal waters.
It is a powerful swimmer with a streamlined body, strongly calcified skeleton, stiff fins, strong skin, and firm muscle and connective tissue (Compango, 1981). A mature male can grow up to 7.5 m or more, cruise at about 2 knots at the surface, and pass an average of 1,650 tons of water an hour through its huge gill rakers (Clark, 1981).

A whale shark, Rhincodon typus, is a strong swimmer who often feeds near the surface and can suck in and filter out water when stationary (Compango, 1981).
In contrast, the megamouth is not a strong swimmer, has a weak body musculature, soft fins, restricted internal gill openings, and jaw morphology does not facilitate efficient feeding by the same method seen by the basking or whale shark (Compango, 1990).
How does the Megamouth Shark Feed?
So how could such a significant but weak animal, with a low-flow filter and living well below the abundant plankton, filter enough plankton to support itself?
Although exact details of the megamouth’s feeding behavior can not be confirmed because no living specimen has been studied, several theories have been discussed; Diamond (1985) suggested that the bright silvery lining of the mouth is bioluminescent.
When the first specimen was taken aboard the U.S. Navy research ship, it was noted the upper jaw and palate had a silvery, iridescent lining, which was questioned if it was luminescent.
Taylor et al. (1983) suggested that the megamouth had a bioluminescent mouth but could not prove it because of poor preservation, but the better preserved Catalina specimen revealed possible luminescent tissue along with iridescent, reflective upper jaw tissue (Taylor et al.., 1983; Lavenburg and Seigal, 1985).
Diamond (1985) contends that the stomach content included species that live deep in the scattering layer and are known to be bioluminescent. Therefore, he believes that the megamouth may be holding its mouth open in the dark, thus attracting plankton-like moths to a “light trap.”
Diamond (1985) adds that no other known plankton feeder is known to utilize this type of mechanism. If evidence is given that the megamouth attracts plankton into their mouths by light, Diamond (1985) raises the question of how this behavior evolved.
Megamouth Feeding Scenarios
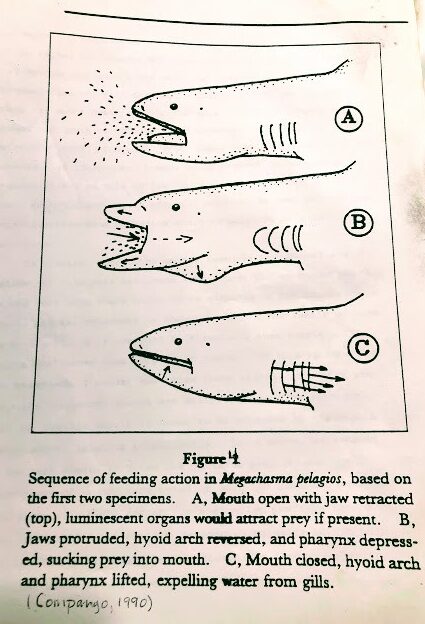
The first feeding scenario was given by Taylor et al. (1983). It suggested that the megamouth ” can be imagined as slow swimming through schools of euphausiid shrimp and possibly other prey with jaws widely opened, occasionally closing its mouth and contracting its pharynx to expel water and concentrate prey before swallowing it,”
Compango (1990) argues this hypothesis through his morphological observations of the first two specimens.
He believes that the Taylor et al. (1983) scenario was influenced by the known feeding habits of the basking shark. Compango’s (1990) observations suggest a revised scenario for feeding the megamouth shark that is consistent with its feeding apparatus and its possible sluggishness.
He suggests that if luminescent tissue is present on the upper jaw., the luminous, reflective tissue may be attractive to potential prey when producing light and may serve to concentrate its prey near its mouth and jaws.
Then suddenly, the shark protrudes its jaws, which depresses its hyoid arch, and drops its tongue and pharynx, significantly increasing its pharynx volume. Like a giant below, it sucks the prey inside (see Fig 4).
The megamouth then closes its mouth and retracts its jaws. This action raises the pharynx, and a huge tongue decreases the pharyngeal volume and expels the water out through its closely screened internal gill openings.
The shark swallows its food, opens its mouth again, and waits for more victims to concentrate around its mouth or slowly swims elsewhere to locate undisturbed patches of prey.
Compango (1990) feels that this scenario is not dependent on luminescent organs being present in Megachasma because it may be able to feed on prey concentrations without their possible attractive effect.
However, he does believe that a luminous oral lure would enhance the Megachasmids’ feeding mechanism.
A Few Other Unique Megachasma pelagios Features
In closing, some other unique features of the megamouth shark to consider are the following:
- Only male specimens were found
- It is the only live shark known to be attacked by the cookiecutter shark
- It is known to host a new family of tapeworm
First, it is reasoned by scientists that female adult sharks are more prominent than male adult sharks. If this is true for the megamouth, I wonder how large these sharks can get.
Secondly, megamouth sharks are believed to be slow swimmers, which may explain why they are known to be attacked by the cookiecutter shark, Isistius brasiliensis.
It is so-called because of the neat pieces it bites out of large prey (Clark, 1981). These wounds were noted on the Hawaiian specimen (Taylor et al.., 1983) and the Australian specimen (Berra and Hutchins, 1990).
A New Tapeworm Too?
And finally, Megachasma pelagios is a host of a new family of tapeworms Mixodigmatidae (order Trypanorhnchida), described by Daily and Vogelbein (1982), for the new genus and species Mixodigma leptaleum.
This parasitic tapeworm from the valvular intestine presented as many problems in placing it in existing trypanorhynch families as did placing Megachasma pelagios in an existing lamnoid family (Taylor et al.., 1983)
Literature Cited
Berra, T.M, and J.B. Hutchins, 1990. A specimen of megamouth shark, Megachasma pelagios (Megachasmidae) from Western Australia. Rec. West Aust. Mus. v40 651-656
Clark, Eugenie, 1981. Sharks: magnificent and misunderstood. National Geographic. 160 (Feb): 138-187
Compango, L.J.V., 1981. Legend versus reality; the Jaws image and shark diversity. Oceanus, v24: 5-16.
Compango, L.J.V., 1983. Sharks of the Order Carcharhiniformes. Princeton, NJ, 580p.
Compango, L.J.V., 1990. Relations of the megamouth shark, Megachasma pelagios (Lamniformes: Megachasmisdae), with particular comments on its feeding habits. NOAA Tep Rep NWFS No.90: 357-379.
Diamond. J.M., 1985. Filter-feeding of a grand scale. Nature, 316: 679-680
Lavenburg R.J., and J.A. Seigal, 1985. The Pacific’s mega mystery- Megamouth. Copela, 1985: 226-231.
Maisey, J.G., 1985. Relationships of the megamouth sharks, Megachasma. Copeia 1985: 228-231
Nakaya, K., 1989. Discovery of megamouth shark from Japan. Jpn J. Ichthyol. 36: 144-146
Springer, V.G., and J.P. Gold. 1989. Sharks in Question. Smithsonian Institution Press, Washington, D.C., pp 103-104.
Taylor, L.R., L.J.V. Compango, and P.J. Struhsaker, 1983. Megamouth- and new species, genus, and family of lamnoid shark (Megachasma pelagios), Family Megachasmidae) from the Hawaiian Islands. Proc. Calif. Acad. Sci. 43: 87-110.
Wood, L., 1986. Megamouth: new species of shark. Sea Frontiers/ Sea Secrets. 32: 192-198.
Updated Megamouth Shark Information!
Dana Point
On October 21, 1991, a male megamouth was tangled in a fishing net off Dana Point, California. Researchers rushed out to sea to document the situation and tagged it. They were able to keep track of the shark for three days. What they learned was fascinating!
The megamouth shark has a vertical migration.
During the day, it would stay below at around 300 feet and ascend at night to depths of only 26ft to 50ft following the krill.
This might be a significant clue as to why the megamouth is rarely seen.
San Diego
On September 11, 2022, two Megamouth sharks were filmed swimming on the surface together 39km off the coast of San Diego. The film from this encounter gave much insight into this elusive creature. Never before has more than one individual been seen together. In fact, only five free swimming individuals have been scientifically recorded.
A recreational fishing vessel, at 11:26 am noticed two dorsal fins breaking the surface 45 to 60 meters from their boat.
So, what did they learn?
Additional Megamouth Sightings in the Last 30 Years

I have always had a deep-seated passion for the Ocean Environment which ultimately led me to receive a degree in Marine Biology. Living in the San Diego area for over 30 years, I have extensively explored the 70 miles of San Diego’s coastline, and I am here to share! Please use my website to your advantage and have a look around at all the wonders that the beaches of San Diego can offer you!

